Proteomics Using Protease Alternatives to Trypsin Benefits from Sequential Digestion with Trypsin
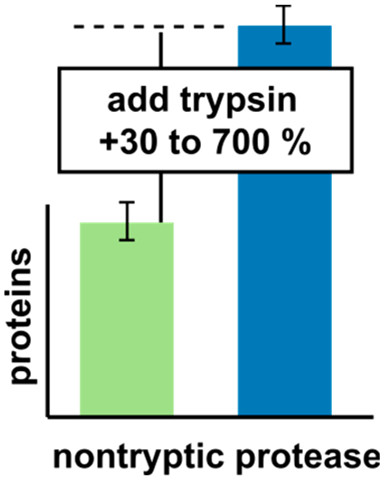
Therese Dau, Giulia Bartolomucci, Juri Rappsilber Analytical Chemistry, 2020 https://doi.org/10.1021/acs.analchem.0c00478 Trypsin is the most used enzyme in proteomics. Nevertheless, proteases with complementary cleavage specificity have been applied in special circumstances. In this work, we analyzed the characteristics of five protease alternatives to trypsin for protein identification and sequence coverage when applied to S. pombe whole cell lysates.
Read More...Ultraviolet Photodissociation of Tryptic Peptide Backbones at 213 nm
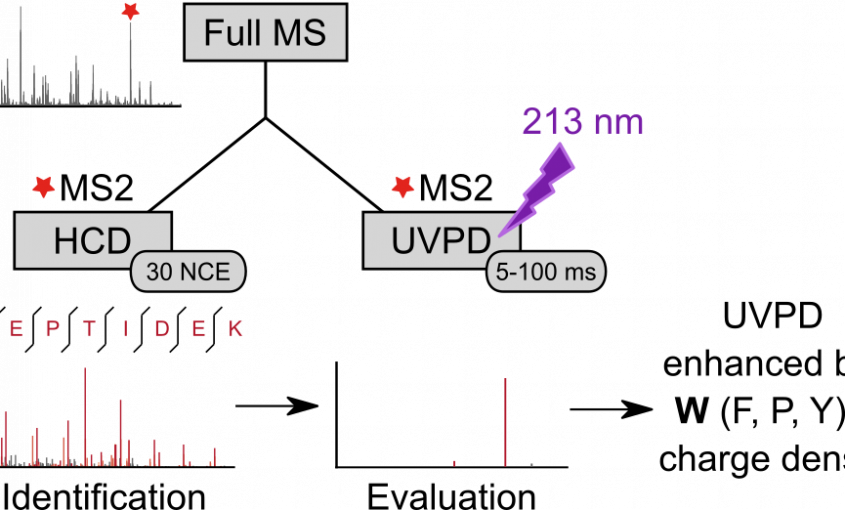
Lars Kolbowski, Adam Belsom, Juri Rappsilber Journal of the American Society for Mass Spectrometry, 2020 https://doi.org/10.1021/jasms.0c00106 We analyzed the backbone fragmentation behavior of tryptic peptides of a four protein mixture and of E. coli lysate subjected to Ultraviolet Photodissociation (UVPD) at 213 nm on a commercially available UVPD-equipped tribrid mass spectrometer. We obtained 15,178 unique
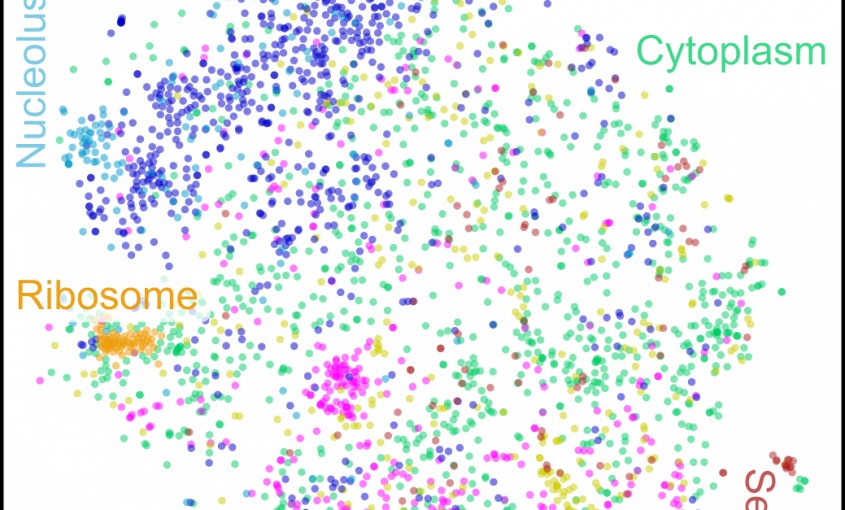
Read More...Co-regulation map of the human proteome enables identification of protein functions
Nature Biotechnology, 2019
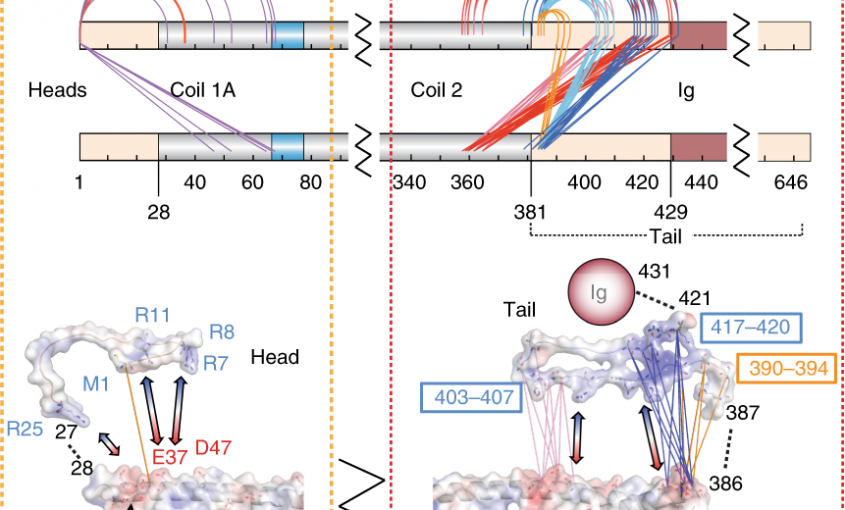
Read More...Lamin A molecular compression and sliding as mechanisms behind nucleoskeleton elasticity
Nature Communications, 2019
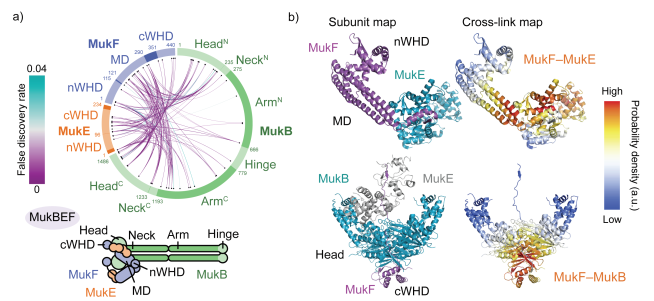
Read More...A folded conformation of MukBEF and cohesin
Nature Structural & Molecular Biology, 2019
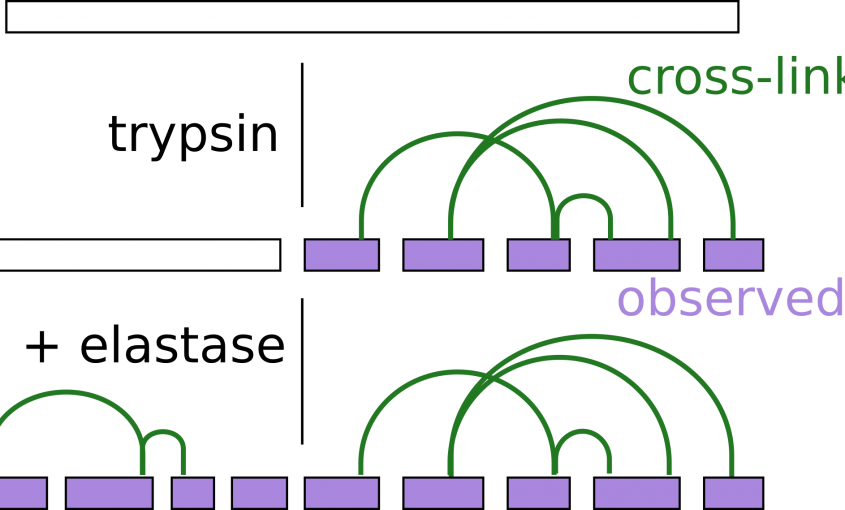
Read More...Sequential Digestion with Trypsin and Elastase in Cross-Linking Mass Spectrometry.
Analytical Chemistry, 2019
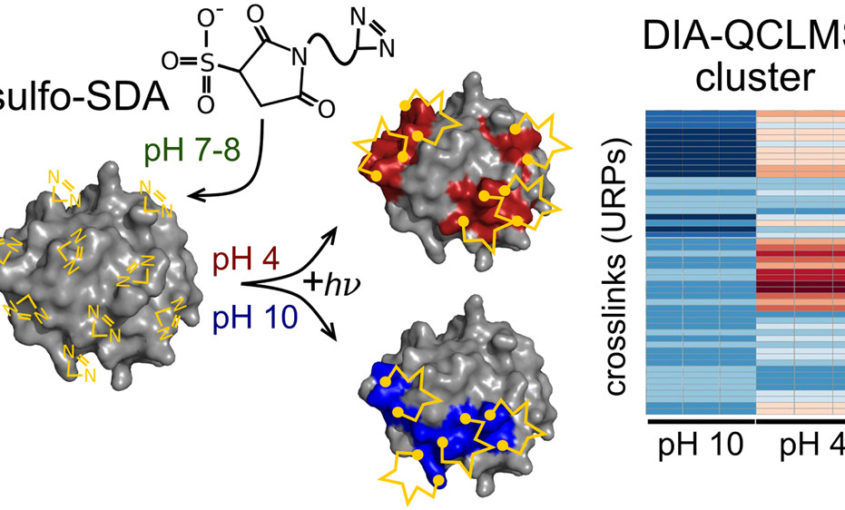
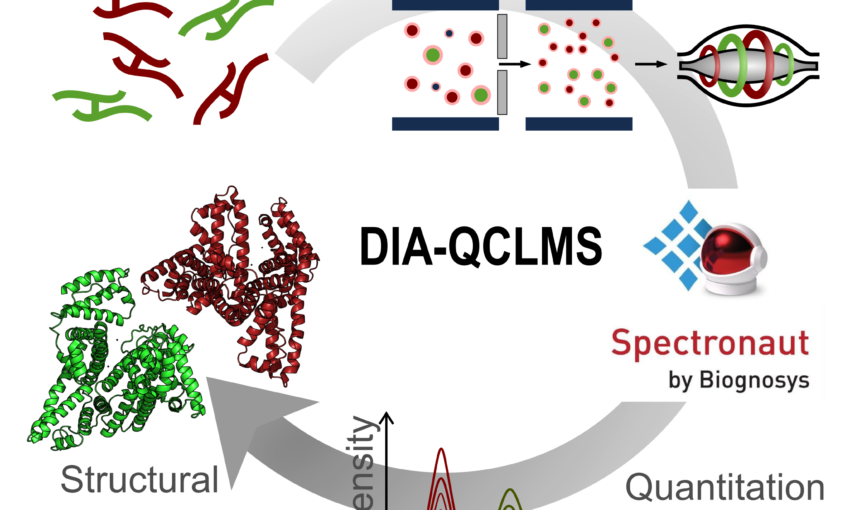
Read More...Data-independent Acquisition Improves Quantitative Cross-linking Mass Spectrometry
Molecular & Cellular Proteomics, 2019
Read More...