10.1021/acs.analchem.6b04935
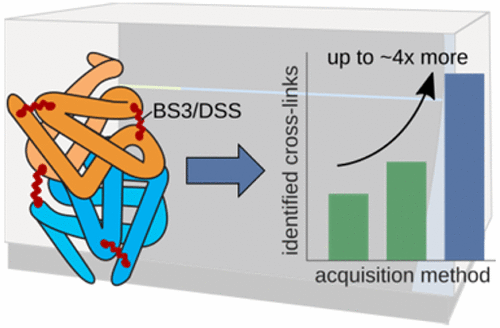
We compared the five different ways of fragmentation available on a tribrid mass spectrometer and optimized their collision energies with regard to optimal sequence coverage of cross-linked peptides. We created a library of bis(sulfosuccinimidyl)suberate (BS3/DSS) cross-linked precursors, derived from the tryptic digests of three model proteins (Human Serum Albumin, creatine kinase, and myoglobin). This enabled in-depth targeted analysis of the fragmentation behavior of 1065 cross-linked precursors using the five fragmentation techniques: collision-induced dissociation (CID), beam-type CID (HCD), electron-transfer dissociation (ETD), and the combinations ETciD and EThcD. EThcD gave the best sequence coverage for cross-linked m/zspecies with high charge density, while HCD was optimal for all others. We tested the resulting data-dependent decision tree against collision energy-optimized single methods on two samples of differing complexity (a mix of eight proteins and a highly complex ribosomal cellular fraction). For the high complexity sample the decision tree gave the highest number of identified cross-linked peptide pairs passing a 5% false discovery rate (on average ∼21% more than the second best, HCD). For the medium complexity sample, the higher speed of HCD proved decisive. Currently, acquisition speed plays an important role in allowing the detection of cross-linked peptides against the background of linear peptides. Enrichment of cross-linked peptides will reduce this role and favor methods that provide spectra of higher quality. Data are available via ProteomeXchange with identifier PXD006131.