Therese Dau, Giulia Bartolomucci, Juri Rappsilber
Analytical Chemistry, 2020
https://doi.org/10.1021/acs.analchem.0c00478
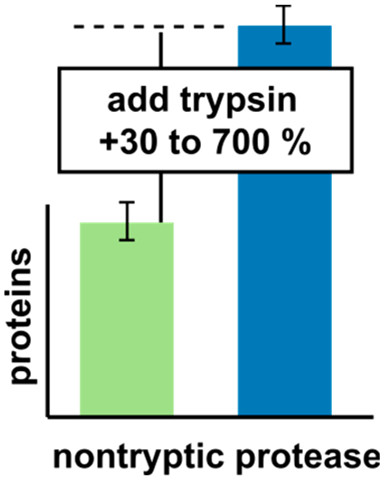
Trypsin is the most used enzyme in proteomics. Nevertheless, proteases with complementary cleavage specificity have been applied in special circumstances. In this work, we analyzed the characteristics of five protease alternatives to trypsin for protein identification and sequence coverage when applied to S. pombe whole cell lysates. The specificity of the protease heavily impacted the number of proteins identified. Proteases with higher specificity led to the identification of more proteins than proteases with lower specificity. However, AspN, GluC, chymotrypsin, and proteinase K largely benefited from being paired with trypsin in sequential digestion, as had been shown by us for elastase before. In the most extreme case, predigesting with trypsin improves the number of identified proteins for proteinase K by 731%. Trypsin predigestion also improved the protein identifications of other proteases, AspN (+62%), GluC (+80%), and chymotrypsin (+21%). Interestingly, the sequential digest with trypsin and AspN yielded even a higher number of protein identifications than digesting with trypsin alone.